When two or more drugs are combined into one pill or formulation, it’s not just about adding doses. It’s about making sure the therapeutic equivalence holds up - even when the doses change, the manufacturers switch, or the inactive ingredients vary. For millions of patients taking combination products like amlodipine/benazepril for high blood pressure or ezetimibe/simvastatin for cholesterol, getting the right dose isn’t optional. It’s life-or-death. And yet, many assume that if two products have the same active ingredients and the same FDA ‘A’ rating, they’re interchangeable without consequence. That’s not always true.
What Therapeutic Equivalence Really Means
Therapeutic equivalence means two drug products can be substituted for each other without changing the patient’s clinical outcome. The U.S. FDA tracks this in the Orange Book, where over 14,000 drugs are rated. Only those with identical active ingredients, dosage forms, strengths, and routes of administration get an ‘A’ rating. That’s the gold standard. But here’s the catch: it doesn’t mean identical results in every person. For example, take the combination of tramadol and acetaminophen. One brand might release tramadol slowly over 12 hours, while a generic version releases it in bursts. Even if both meet bioequivalence standards (80-125% absorption range), the timing of pain relief can shift. That’s not a flaw in the system - it’s a limitation. The FDA doesn’t test for every possible patient response. It tests for population averages.Different Doses, Same Rating? Here’s Where It Gets Tricky
Combination products often come in multiple strengths. Amlodipine/benazepril, for instance, is available as 5/10mg, 10/20mg, and 10/40mg. If a pharmacist switches a patient from 5/10mg to 10/20mg because the other is out of stock, they’re doubling the dose of both drugs. That’s not therapeutic equivalence - that’s a dosing error. But if the switch is between two different brands of the same strength, say 10/20mg, then it’s supposed to be safe. The problem? Not all manufacturers produce the same release profile. A 2023 study found that among seven generic versions of rivaroxaban (a blood thinner), three used croscarmellose sodium as a disintegrant, while four used sodium starch glycolate. These differences can affect how quickly the drug dissolves in the gut - especially when combined with another medication that alters stomach pH. For a patient on rivaroxaban plus an NSAID, even a 10% delay in absorption can raise the risk of clotting or bleeding.Narrow Therapeutic Index Drugs: The Hidden Danger Zone
Some drugs are like walking a tightrope. Too little, and they don’t work. Too much, and they poison you. These are called narrow therapeutic index (NTI) drugs. Examples include warfarin, levothyroxine, phenytoin, and lithium. When they’re part of a combination - say, levothyroxine plus a calcium supplement that interferes with absorption - things get even more fragile. The FDA requires stricter bioequivalence standards for NTI drugs: 90-111% instead of the usual 80-125%. But even that isn’t foolproof. A 2018 study in the Journal of Clinical Endocrinology & Metabolism found that 12% of patients switching between different levothyroxine generics had abnormal thyroid levels within six weeks - despite all products meeting FDA standards. One patient went from feeling fine to experiencing heart palpitations and weight loss. Their TSH levels jumped from 2.1 to 8.7 mIU/L. That’s not a small change. It’s a clinical emergency. And it’s not just thyroid meds. Psychiatric combinations - like sertraline plus olanzapine - show similar issues. Pharmacokinetic interactions between components can change how each drug is metabolized. A patient stable on one brand might crash on another, not because the dose is wrong, but because the formulation altered the drug’s behavior in the body.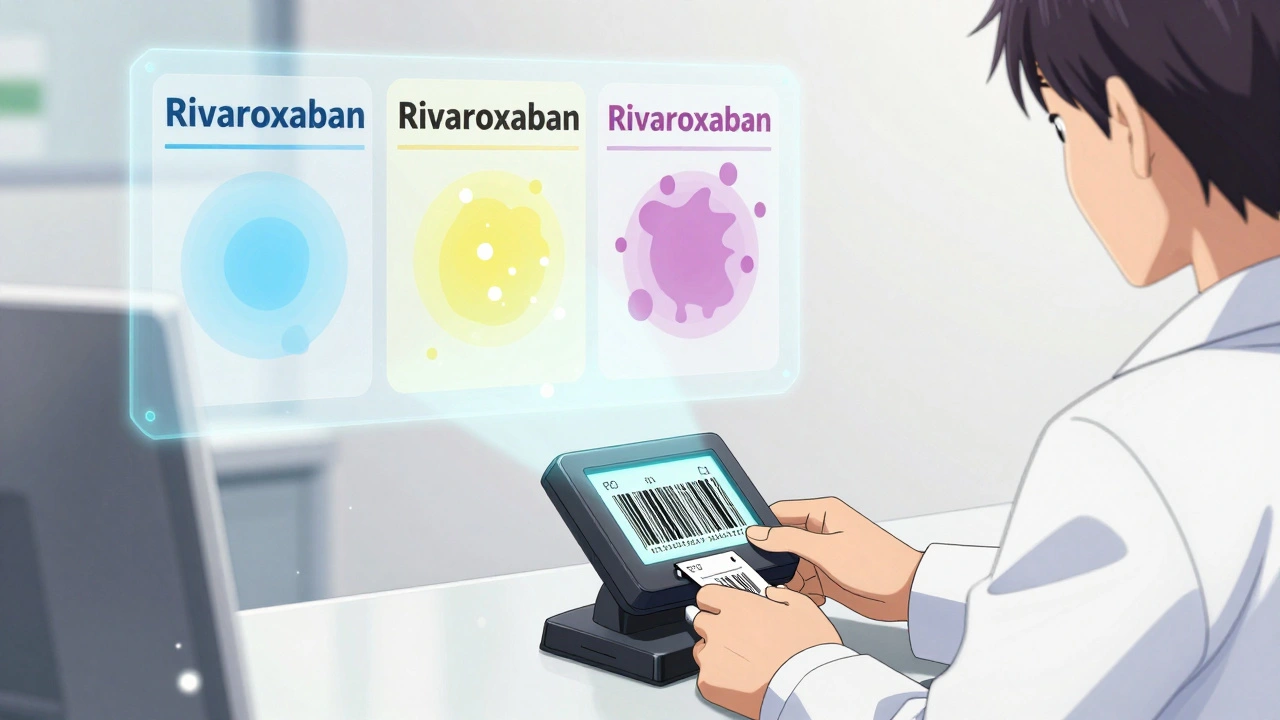
Why Generic Substitution Can Backfire in Combination Products
The big promise of therapeutic equivalence is cost savings. The generic version of Advair Diskus (fluticasone/salmeterol) costs 40% less than the brand and has a 97% equivalence rating. That sounds perfect. But here’s what’s rarely said: that 3% difference matters. For a COPD patient on high-dose steroids, even a 3% drop in lung deposition can mean more flare-ups. Real-world data backs this up. On Reddit, a pharmacist with 12 years of experience reported three dosing errors in six months from switching between different strengths of amlodipine/benazepril. One patient was accidentally given 10/40mg instead of 5/20mg. They ended up in the ER with dangerously low blood pressure. Another case on Allnurses.com described a patient whose LDL cholesterol jumped 15% after switching from brand-name Vytorin to a generic - not because of the active ingredients, but because of the filler that slowed absorption. The FDA’s Adverse Event Reporting System logged 247 incidents in 2022 tied to dose conversion errors in combination products. Nearly 40% involved cardiovascular drugs. Another 30% involved psychiatric meds. These aren’t rare. They’re systemic.How to Manage This Safely
There’s no magic fix. But there are steps that reduce risk:- Check the TE code - not just the name. Make sure it’s ‘A’ and the strength matches exactly.
- Don’t assume equivalence across strengths - switching from 5/10mg to 10/20mg is doubling the dose. That’s not substitution. That’s a new prescription.
- Watch for NTI drugs - if any component is warfarin, levothyroxine, or phenytoin, treat substitution like a high-risk procedure. Monitor labs for 4-6 weeks after the switch.
- Use barcode scanning - many hospitals now require scanning both the prescription and the dispensed product. This cuts substitution errors by over 60%.
- Keep a 72-hour observation window - especially for patients on multiple NTI drugs or with kidney/liver issues. Watch for subtle signs: fatigue, dizziness, mood changes, or irregular heartbeat.
What’s Changing in 2025
The FDA is rolling out new tools. In early 2023, they released draft guidance for complex combination products - those where dose-response isn’t linear. For example, some combinations show synergistic effects (like tramadol + acetaminophen), meaning 50mg of each isn’t just half the effect of 100mg of one. It’s more. The agency is now using machine learning models to predict when formulation changes might break therapeutic equivalence. Early tests hit 89% accuracy. There’s also talk of an ‘A*’ rating - a new category for combination products that prove bioequivalence across multiple strengths. Right now, each strength is rated separately. That’s inefficient and confusing. And long-term? The NIH predicts that by 2030, 30% of therapeutic equivalence decisions will include pharmacogenomic data. That means a patient’s DNA could determine whether a generic version is safe for them. Someone with a slow-metabolizer CYP2D6 gene might need a different formulation than someone who clears drugs quickly. This isn’t sci-fi. It’s already being tested in clinical trials.Bottom Line: Equivalence Isn’t Always Equal
Therapeutic equivalence is a powerful tool. It saves billions. It makes medicines accessible. But it’s not a guarantee. For combination products, especially those with NTI components or complex interactions, the difference between ‘equivalent’ and ‘safe’ can be tiny - and deadly. If you’re prescribing, dispensing, or taking combination drugs, don’t treat therapeutic equivalence like a checkbox. Treat it like a conversation - with your pharmacist, your doctor, and your body. If you switch brands and feel different, don’t ignore it. Document it. Report it. Your safety isn’t just about the dose on the label. It’s about how your body responds to every ingredient - visible and invisible.Are all generic combination drugs therapeutically equivalent to the brand?
No. Only those with an ‘A’ rating in the FDA’s Orange Book are considered therapeutically equivalent. Even then, differences in inactive ingredients, release profiles, or manufacturing can affect how the drug performs in individual patients - especially with narrow therapeutic index drugs like warfarin or levothyroxine.
Can I switch between different strengths of a combination drug if they have the same TE code?
Never. Different strengths are not interchangeable. For example, switching from amlodipine/benazepril 5/10mg to 10/20mg doubles the dose of both components. This is a medication error, not a substitution. Always verify the exact strength matches the prescription.
Why do some patients have bad reactions after switching to a generic combination drug?
Even if two products have identical active ingredients and an ‘A’ rating, differences in fillers, coatings, or release mechanisms can alter absorption. This is especially risky with NTI drugs or when combining multiple medications that interact. One patient might metabolize the drug normally, while another - due to genetics, age, or liver function - experiences a spike in blood levels or delayed effect.
How do I know if my combination drug is a narrow therapeutic index (NTI) drug?
Common NTI drugs include warfarin, levothyroxine, phenytoin, lithium, cyclosporine, and digoxin. If your combination includes any of these, treat any switch with caution. Ask your pharmacist or doctor for a list of NTI drugs in your regimen. The FDA and ISMP provide official lists for healthcare providers.
What should I do if I notice a change in how I feel after switching combination drugs?
Don’t wait. Contact your prescriber immediately. Keep a log of symptoms - timing, severity, and any other medications taken. Report the incident to the FDA’s MedWatch program. Many adverse events go unreported, but your report helps improve safety for others. In the meantime, avoid further substitutions until you’ve been evaluated.


Olivia Hand
December 6, 2025 AT 18:40So let me get this straight - the FDA says two drugs are ‘equivalent’ if they’re within 80-125% absorption, but a 10% delay in dissolution can kill someone on rivaroxaban? That’s not equivalence. That’s Russian roulette with a prescription.
I’ve seen patients crash after generic switches and been told, ‘It’s the same chemical.’ No. It’s the same *label*. The body doesn’t read labels. It reads bioavailability. And that’s where the system fails.
And don’t even get me started on how pharmacists are pressured to swap for cost savings. It’s not substitution - it’s gambling with someone’s heartbeat.
Sangram Lavte
December 7, 2025 AT 17:48This is exactly why I always write down the manufacturer name when I pick up my meds. Last month, my levothyroxine switched from Teva to Mylan - I felt like I’d been drugged. Tired all day, heart racing. Went back to the pharmacy, showed them the bottle. They said, ‘Same active ingredient.’ I said, ‘Yeah, and so is water and vodka.’
They gave me my old brand back. No questions. Maybe we need a barcode system that tracks manufacturer, not just strength.
Stacy here
December 8, 2025 AT 12:43THEY KNOW. THEY KNOW THIS IS A SCAM.
Big Pharma doesn’t want you to know that generics can kill. Why? Because they sold the patents. Now they profit from the brand name *and* the FDA’s broken system. The ‘A’ rating? A marketing tool. The real test? Does your body survive the switch?
And don’t tell me ‘clinical trials prove safety.’ Trials are done on healthy 30-year-olds. Not on 72-year-olds with kidney failure taking 5 meds.
They’re testing drugs like they’re video game skins. ‘Looks the same, works the same.’ Except your body isn’t a skin. It’s a temple. And they’re turning it into a lab rat.
They’re coming for your thyroid next. Then your heart. Then your brain. Mark my words.
Kyle Flores
December 9, 2025 AT 03:45I work in a clinic and I’ve seen this firsthand. One woman switched from brand to generic amlodipine/benazepril and started passing out in the waiting room. We thought it was anxiety. Turns out, her BP dropped to 78/45. She didn’t know the strength changed - the pharmacist didn’t tell her.
Now we have a checklist we print out for every combo med. Manufacturer, strength, TE code. We hand it to the patient. We make them read it out loud.
It’s not fancy. But it saves lives. And honestly? If your pharmacy won’t do that, find a new one. Your life isn’t a cost-saving experiment.
Louis Llaine
December 10, 2025 AT 00:05Wow. So the FDA’s ‘gold standard’ is basically ‘close enough for government work.’
Let me get this: we’re trusting our lives to a statistical range of 80-125% absorption? That’s like saying two cars are ‘equivalent’ if one goes 0-60 in 6 seconds and the other in 10. Sure, both are ‘cars.’ But one’s a Lambo and the other’s a broken Prius.
Also, who’s paying for the 247 ER visits? Not the generic makers. Not the FDA. Just us. Thanks, capitalism.
Jane Quitain
December 11, 2025 AT 15:29Y’all need to stop being scared and start being proactive!! 🌟
If you feel weird after a switch, JOURNAL IT. Write down the date, the med, the manufacturer, how you felt. Then send it to the FDA. Seriously. I did it after my mood crashed post-generic sertraline switch - they actually called me back!
And if your pharmacist doesn’t explain the TE code? Find one who does. You’re worth the extra 5 minutes. You’re worth your life. 💪❤️
Ernie Blevins
December 11, 2025 AT 15:58People are dying because they’re too lazy to read the label.
It’s not the system’s fault. It’s yours. If you don’t know what’s in your pill, you deserve what happens.
Also, why are you even on combo drugs? Just take two pills. It’s not that hard. Stop being a baby.
Nancy Carlsen
December 12, 2025 AT 12:29This hit me right in the feels 😢
My mom switched generics for her levothyroxine and went from hiking every weekend to barely getting out of bed. We didn’t connect the dots until her TSH was through the roof.
But here’s the good news - once we switched back, she was herself again. 💛
So if you’re reading this - please, please, please keep track of your meds. Write the manufacturer on your pillbox. Tell your doctor. You’re not overreacting. You’re being smart. And you’re not alone. We got you 💕
Ashley Farmer
December 13, 2025 AT 21:56I’m a nurse and I’ve seen this too many times. A patient comes in confused, tired, anxious - and we find out they were switched to a different generic of their combo med. No warning. No follow-up.
It’s not just about the drug. It’s about the trust. When you’re told ‘it’s the same,’ you believe it. And when your body betrays you, you feel broken.
Let’s stop normalizing this. Ask for the TE code. Ask for the manufacturer. Ask for time to adjust. You have the right to ask.
Sadie Nastor
December 14, 2025 AT 22:02my doc just switched me to a generic combo and i felt like i was drunk for 3 days 😅
not even tipsy - like, my brain was slow. foggy. i thought it was stress. turns out the new generic had a different filler that slowed absorption. took 2 weeks to feel normal again.
now i write the maker on my calendar. and i tell every friend who takes meds to do the same. it’s tiny, but it helps. ❤️
Kurt Russell
December 15, 2025 AT 23:50THIS IS THE MOST IMPORTANT THING YOU’LL READ THIS YEAR.
Let me tell you about my cousin. 58. On warfarin + aspirin combo. Switched generics. INR spiked to 8.2. Bleeding in the brain. Coma. Survived. But now he can’t walk right. Lost his job. Lost his independence.
It wasn’t ‘bad luck.’ It was systemic negligence.
They’re saving pennies on pills and losing years of human life.
If you’re reading this - speak up. Report it. Don’t let this be someone’s story. Be the person who says: ‘Not on my watch.’
Kyle Oksten
December 16, 2025 AT 20:38Therapeutic equivalence is a myth built on the assumption that biology is linear. It’s not. Humans aren’t test tubes. We’re messy, genetically unique, metabolically chaotic systems.
The FDA tests for population averages. But your life isn’t an average. It’s a singularity.
So when they say ‘equivalent,’ what they really mean is: ‘We’ve done enough to satisfy regulators, not patients.’
And until we treat the body like a sacred, individual ecosystem - not a statistical variable - this will keep happening.
Helen Maples
December 17, 2025 AT 14:35Every single pharmacist and prescriber needs to be trained on this. Not ‘awareness.’ TRAINING.
TE codes aren’t optional. NTI flags aren’t suggestions. Manufacturer tracking isn’t ‘extra.’ It’s standard of care.
I’ve seen EHRs that don’t even alert for NTI drugs. That’s malpractice. Not negligence. Malpractice.
If your clinic doesn’t have a combo drug protocol, demand one. Or find a new provider. Your life isn’t a spreadsheet.
David Brooks
December 18, 2025 AT 21:01I used to think generics were just cheaper. Now I know they’re a gamble.
My dad was on simvastatin/ezetimibe. Switched to generic. LDL jumped 20%. He didn’t even know. His doctor didn’t check.
So now? I check his labs every 4 weeks after any switch. I call the pharmacy. I write down the lot number.
It’s exhausting. But I’d rather be annoying than bury my dad because someone thought ‘close enough’ was good enough.
Jennifer Anderson
December 20, 2025 AT 19:32just started taking a combo med and i had no idea about te codes 😳
now i google every pill i get. i write the maker on my phone. i ask the pharmacist to point it out. its crazy how little info you get.
thanks for this post - i feel less alone now. 💕