When you see a pill that looks exactly like the brand-name drug you’ve been taking - same color, same shape, same imprint - but it costs half as much, you might assume it’s a generic made by a different company. But what if that same pill was made in the exact same factory, with the exact same ingredients, by the same company that made the brand version? That’s not a trick. It’s called an authorized generic, and it’s a common, well-documented strategy used by major drug makers to protect their profits when patents expire.
What Exactly Is an Authorized Generic?
An authorized generic is a version of a brand-name drug that’s produced and sold by the original manufacturer, but without the brand name on the label. It’s not a copy. It’s the same drug, made on the same production line, with the same inactive ingredients, the same quality controls, and the same FDA approval as the brand version. The only differences? The packaging and the price. The first authorized generic launched in 1997 when AstraZeneca released a generic version of its heartburn drug Prilosec (omeprazole) on the day the patent expired. Within six months, it captured 30% of the entire omeprazole market. Since then, this strategy has become standard practice. Between 2018 and 2022, 68% of the top 50 brand-name drugs that lost patent protection got an authorized generic version from their original maker.Why Do Companies Make Their Own Generics?
When a drug’s patent runs out, generic competitors flood the market. Prices can drop 80-85% within the first year. That’s a massive hit to revenue. So instead of watching their customers switch to cheaper alternatives made by others, brand manufacturers create their own generic version to keep those customers - and their profits - from slipping away. By launching an authorized generic, companies can:- Keep control over production and quality
- Block competitors from capturing the entire generic market
- Charge slightly less than the brand version but still more than other generics - creating a middle tier that feels like a discount but still protects margins
How Is It Made? The Production Process
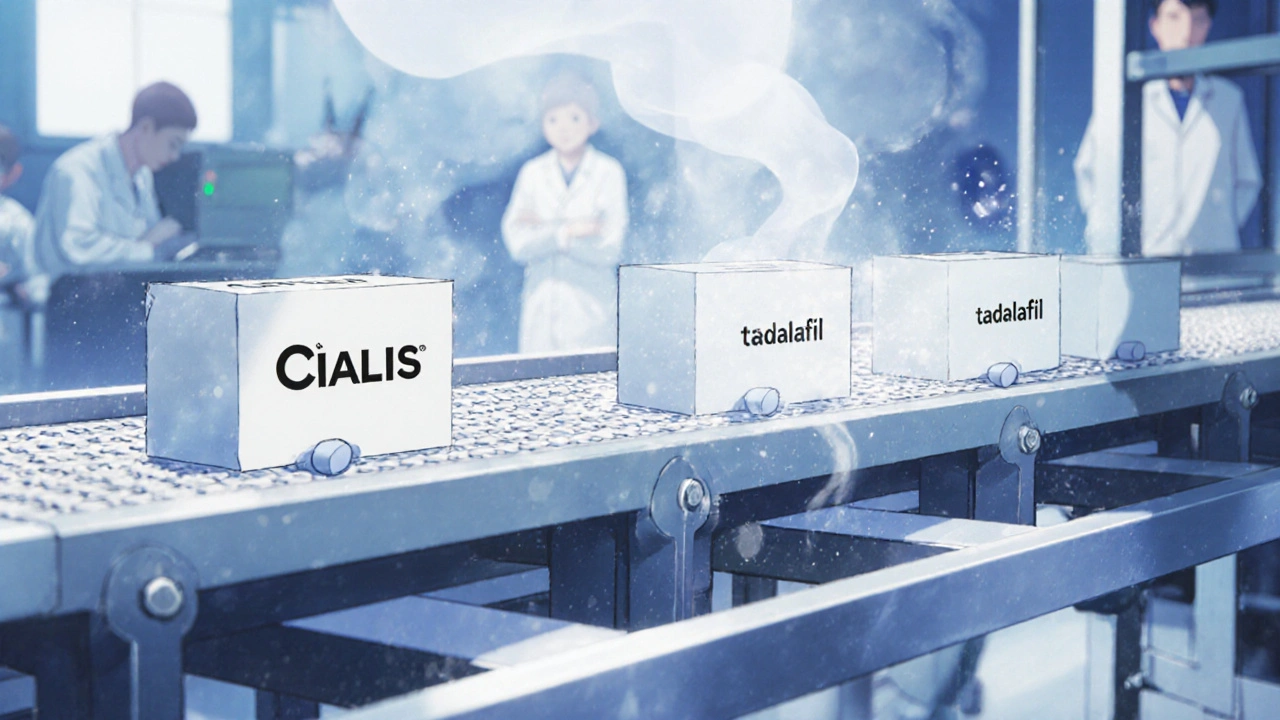
There’s no need to rebuild the factory or retest the drug. The brand manufacturer already has everything they need: the formula, the equipment, the FDA approvals, and the manufacturing license. All they have to do is switch the label. The FDA allows brand manufacturers to use their existing Abbreviated New Drug Application (ANDA) to launch an authorized generic. That means no new clinical trials, no new inspections, and no waiting for 17 months like traditional generic companies do. The whole transition usually takes 6-9 months - mostly just for packaging changes and regulatory paperwork. The same machines, the same chemists, the same quality checks. One batch of pills might go into blue capsules labeled "Cialis," and the very next batch goes into white capsules labeled "tadalafil" - same drug, different box.
How It Affects Pricing and Competition
Authorized generics don’t always save you money. In fact, they often don’t. Traditional generics, made by companies like Teva or Mylan, typically sell for 80-90% less than the brand. But authorized generics? They’re usually priced 10-15% below the brand but 5-10% above other generics. So if the brand costs $120, the authorized generic might be $105, while a regular generic is $35. This creates a confusing market. Patients think they’re getting a "generic" discount, but they’re still paying more than they could. A 2023 Reddit thread on r/pharmacy had over 140 comments - many users were shocked to learn their "generic" was made by the same company as the brand, and still cost $85 when a true generic was $30. But here’s the twist: authorized generics still reduce overall drug spending. The Congressional Budget Office estimates they save $2.3 billion a year compared to no generics at all. The problem? They block the deeper price drops that come from real competition.Regulatory and Legal Controversies
This strategy isn’t illegal - but it’s been controversial. The Federal Trade Commission (FTC) has sued several drug companies for using authorized generics to delay competition. In 2017, the FTC won a $448 million settlement against Actavis for launching an authorized generic of Namenda (memantine) the same day the patent expired. The agency argued this was a tactic to scare off other generic makers from entering the market. The FTC claims that when a brand manufacturer controls both the brand and the "generic," it reduces true competition. The Hatch-Waxman Act of 1984 created the legal framework for generics - including a 180-day exclusivity period for the first generic applicant. But authorized generics bypass that rule entirely. The brand company doesn’t need to wait. They can launch on day one, even if another company has the legal right to be first. In 2019, Mylan and GlaxoSmithKline got into a legal battle over Advair. Mylan had filed to launch a generic, but GSK launched its own authorized version first, delaying Mylan’s entry by eight months. Courts eventually sided with GSK, saying it was legal.
What Do Patients Think?
Patients often don’t know the difference. A 2023 Kaiser Family Foundation survey found that 71% of people preferred authorized generics - but 64% didn’t realize they were made by the same company as the brand. On Drugs.com, authorized generics get higher ratings (4.2/5) than traditional generics (3.8/5). Why? Because patients say: "It’s the same pill I’ve been taking for years. No side effects, no surprises." But independent pharmacists report confusion. A 2022 survey by the National Community Pharmacists Association found that 63% of pharmacy owners said patients often ask: "Why is this generic so expensive?" - not realizing it’s the same drug they’ve always taken.The Bigger Picture: Where This Is Going
The pharmaceutical industry is changing. Over $250 billion worth of brand-name drugs will lose patent protection between 2023 and 2027. And more of those will get authorized generics. Big companies like Pfizer, Johnson & Johnson, and AbbVie are getting smarter about lifecycle management. Instead of waiting for the patent to expire, they start planning 2-3 years ahead. They set up separate marketing teams, repackage inventory, and train pharmacy distributors to handle both versions. And now, this strategy is moving into more complex drugs. In 2023, Amgen launched the first authorized biosimilar - a version of its own biologic drug Enbrel. Biosimilars are harder to copy than pills. They’re made from living cells, not chemicals. Only the original maker has the know-how. So authorized biosimilars might become the new norm for expensive injectables. Analysts predict that by 2027, 25-30% of the entire generic drug market will be made up of authorized generics - up from 18% in 2022.What This Means for You
If you’re paying for a prescription, here’s what you need to know:- Just because it says "generic" doesn’t mean it’s the cheapest option.
- Check the manufacturer name on the bottle. If it’s the same as the brand, you’re looking at an authorized generic.
- Ask your pharmacist: "Is there a traditional generic available?" Often, it’s significantly cheaper.
- Authorized generics aren’t bad - they’re just not always the best deal.
Are authorized generics the same as the brand-name drug?
Yes. Authorized generics are chemically and physically identical to the brand-name drug. They’re made in the same factory, with the same ingredients, and the same quality controls. The only differences are the packaging and the price.
Why are authorized generics more expensive than other generics?
Because they’re not meant to be the cheapest option. Brand manufacturers price them just below the original drug to keep customers from switching to competitors. They’re a middle-ground option - cheaper than the brand, but more expensive than true generics made by other companies.
Can I trust an authorized generic?
Absolutely. Authorized generics are held to the same FDA standards as the brand-name version. In fact, because they’re made by the original manufacturer, many patients report fewer side effects or better consistency compared to generics from unknown companies.
How do I know if my prescription is an authorized generic?
Check the manufacturer name on the bottle or packaging. If it’s the same as the brand-name drug - for example, "Cialis" and "tadalafil" both made by Eli Lilly - it’s an authorized generic. You can also ask your pharmacist directly.
Do authorized generics reduce drug costs overall?
Yes, but not as much as true generic competition. The Congressional Budget Office estimates authorized generics save $2.3 billion annually. But markets with only traditional generics see price drops of 68%, while markets with authorized generics see only 32% drops. They help, but they also slow down deeper savings.

Georgia Green
November 17, 2025 AT 11:20Just learned this yesterday and my mind is blown. I’ve been taking the "generic" omeprazole for years thinking I was saving money - turns out it was made by AstraZeneca the whole time. My pharmacist never mentioned it. I feel like I got played.
Turns out the "cheap" one I buy is $45 at my pharmacy, but the true generic from Teva is $12 at Walmart. Why don’t they tell us this? Is it legal to hide this?
Ashley Unknown
November 19, 2025 AT 07:56Oh wow. So this is why Big Pharma has been so quiet about the "generic" crisis. They didn’t lose the market - they just rebranded it under a different label and kept the same factory running. This isn’t capitalism, this is psychological warfare.
They know we’re conditioned to trust the brand. So they give us the same damn pill in a plain box and call it "generic" - but price it just low enough to feel like a win. Meanwhile, real generics are out here trying to compete and getting crushed because patients are too lazy to check the manufacturer name.
And don’t even get me started on the FTC lawsuits. They’re like cops showing up after the bank’s already been robbed. This has been happening since 1997 and nobody did anything until the profits got too obvious. This system is rigged. The FDA is complicit. The pharmacists are paid to stay quiet. We’re all just lab rats in a pill factory.
I just checked my bottle - it says "Eli Lilly" on the back. I’ve been paying $90 for Cialis for five years. The real generic? $28. I’m switching tomorrow. And I’m telling everyone I know.
They call this innovation? It’s manipulation. And they’re laughing all the way to the bank while we’re over here wondering why our prescriptions keep getting more expensive.
PS: If you’re reading this and you’re on a chronic med - go check your bottle RIGHT NOW. I dare you. I bet you’re getting scammed too.
Margo Utomo
November 20, 2025 AT 14:29Y’all need to stop panicking 😅
Authorized generics are still FDA-approved, same exact pill, just cheaper than brand. If you’re paying $90 for Cialis and there’s a $30 true generic - sure, grab that. But if your doc prescribed it for a reason and you’ve never had side effects? The authorized one is totally fine.
Also - your pharmacist isn’t hiding anything. They’re just busy. Ask them next time! 💬🩺
And yes, the system’s weird. But blaming Big Pharma for being profitable is like blaming a baker for selling bread. They’re not evil. They’re just… business people. 🤷♀️
Sylvia Clarke
November 22, 2025 AT 12:41Let’s not romanticize the "true generic" as some heroic underdog. The reality is that many small generic manufacturers cut corners - not because they’re evil, but because margins are razor-thin. I’ve seen pills with inconsistent dissolution rates, fillers that cause GI distress, and even color variations that trigger anxiety in patients who rely on visual cues.
Authorized generics solve a real problem: consistency. Patients aren’t irrational for preferring them. They’re just trying to avoid the psychological burden of wondering if today’s pill will make them feel like a zombie.
That said - yes, the pricing strategy is predatory. But the solution isn’t to ban authorized generics. It’s to force transparency: mandatory manufacturer labeling on all prescriptions, plus public pricing dashboards. The FTC should mandate this, not just sue after the fact.
And no, this isn’t a conspiracy. It’s just how capitalism works when regulation is reactive, not proactive. We need smarter policy, not outrage.
Matt Wells
November 22, 2025 AT 16:40It is both a legal and economically rational strategy for pharmaceutical manufacturers to introduce authorized generics upon patent expiry. The practice is neither nefarious nor anomalous; it is a predictable consequence of the Hatch-Waxman Act’s structural incentives. The Federal Trade Commission’s interventions, while well-intentioned, often conflate market dynamics with anticompetitive behavior.
One must distinguish between the *production* of a generic and the *market entry* of a generic. The former is a technical act; the latter is a commercial decision. The original manufacturer, possessing the full regulatory dossier and manufacturing infrastructure, is under no obligation to relinquish market share merely because the patent has expired. To do so would be economically irrational.
That patients perceive a psychological benefit from the familiar formulation is not a flaw in the system - it is a feature of human behavior. Behavioral economics demonstrates that brand loyalty persists even in the absence of pharmacological difference. This is not deception; it is consumer preference.
One should not confuse moral outrage with economic insight.
Christina Abellar
November 23, 2025 AT 21:09Good info. I didn’t realize this was so common. Next time I get a prescription, I’ll check the manufacturer. If it’s the same as the brand, I’ll ask if there’s a cheaper one. Simple.
Thanks for breaking this down clearly.
George Gaitara
November 25, 2025 AT 01:56So you’re telling me I’ve been paying $100 for a pill that’s literally identical to the one I could get for $30? And the company that made the original just slapped a new label on it? This isn’t capitalism - this is theft dressed up as a business model.
And don’t give me that "it’s legal" crap. That’s like saying it’s legal to sell someone a fake Rolex and call it "authentic" because you used the same gold.
Why isn’t this illegal? Who’s protecting these companies? Congress? The FDA? The same people who let Purdue Pharma get away with OxyContin?
I’m not mad. I’m just disappointed. We’ve been had.
Deepali Singh
November 25, 2025 AT 10:08Authorized generics are a classic case of rent-seeking behavior disguised as consumer choice. The real cost isn’t in production - it’s in regulatory capture. The FDA’s ANDA pathway, while efficient, was never meant to be weaponized by originators to preempt market entry.
When a firm controls both the branded and "generic" version, it eliminates price competition at the margin. The result? A pseudo-monopoly with two price tiers: one for the gullible, one for the informed.
What’s worse? This strategy is scalable. Now we’re seeing it in biosimilars. The same playbook: same biologic, same facility, same patent cliff exploitation. This isn’t innovation. It’s exploitation with a compliance badge.
Jennifer Howard
November 27, 2025 AT 07:42It is an absolute disgrace that the American public is being systematically deceived by pharmaceutical conglomerates under the guise of "generic" pricing. The fact that patients are unaware that their "discount" medication is manufactured by the very entity that charged them $120 for the same pill just months prior is a moral abomination.
There is no justification for this practice. None. It is a calculated, predatory scheme that preys on the elderly, the chronically ill, and those without the time or resources to research their prescriptions. The FDA should be ashamed. Pharmacists should be required to disclose this at the point of sale. And Congress - Congress is complicit.
When I was prescribed my statin, I trusted the label. I trusted the system. I trusted that the word "generic" meant something. It does not. It means "pay more than you should, because you’re too tired to fight."
And now I know. And I will not be silent.
Abdul Mubeen
November 28, 2025 AT 22:25This is not a market failure. It is a systemic one. The entire regulatory architecture of pharmaceuticals in the United States has been designed to favor incumbent manufacturers. The authorized generic is not an anomaly - it is the logical endpoint of a system that conflates intellectual property with market control.
When a company can legally produce its own generic on day one, it negates the very purpose of the 180-day exclusivity granted to first-filers under Hatch-Waxman. This is not competition. It is regulatory arbitrage.
And let us not forget: the same corporations that deploy this tactic are the ones lobbying against drug price negotiation. They profit from the status quo. They fund the politicians who preserve it. And they profit from the ignorance of the patient.
This is not capitalism. It is corporatism.
Joyce Genon
November 29, 2025 AT 16:09Let me get this straight - the same company that made the brand-name drug for $120 turns around and sells the exact same pill as a "generic" for $105, and somehow that’s supposed to be good for consumers? No. That’s a tax on naivety.
And don’t even get me started on the "it’s the same pill" argument. So what? The same company also makes a $500 bottle of water labeled "Premium Hydration" and sells it next to the $1 store brand. Is that okay too? No. Because context matters.
People don’t care that it’s the same pill. They care that they’re being tricked into paying more than necessary. And the fact that 71% of patients prefer authorized generics proves nothing except that most people don’t know what they’re paying for.
This isn’t a market efficiency. It’s a behavioral trap. And the pharmaceutical industry is the puppet master.
Meanwhile, the real generics? The ones made by Teva, Sandoz, or Mylan? They’re being squeezed out because the system rewards deception over competition. That’s not capitalism. That’s a rigged game.
And now they’re doing this with biosimilars? Great. So next we’ll have "authorized biosimilars" for $2000 that cost $1000 to make, and we’ll all be told to be grateful we’re not paying $3000.
I’m not even mad. I’m just exhausted.
Eva Vega
November 29, 2025 AT 20:18From a regulatory standpoint, the authorized generic mechanism is an elegant exploitation of the ANDA pathway. The original sponsor leverages its pre-existing NDA data and manufacturing compliance to bypass the typical 17-month lead time required for third-party generics. This is not a loophole - it is a statutory feature.
However, the economic implication is a suppression of price elasticity. The presence of an authorized generic reduces the number of independent entrants, thereby dampening the downward pressure on prices that typically follows patent expiry. This results in a suboptimal welfare outcome - higher consumer expenditure than would occur under pure competition.
The FDA’s role is to ensure safety and efficacy, not market structure. That responsibility falls to the FTC and DOJ - and their enforcement has been historically tepid. The 2017 Actavis settlement was a rare exception, not a precedent.
Policy reform should focus on mandating disclosure of manufacturer identity on all prescription labels and prohibiting originators from launching authorized generics within the first 90 days of patent expiry - thereby preserving the incentive structure intended by Hatch-Waxman.